2014-2018 Overview
In Ontario, there are 159 hospitals that transfuse blood products. All TTISS participating hospitals submit reportable ATEs (moderate to severe).
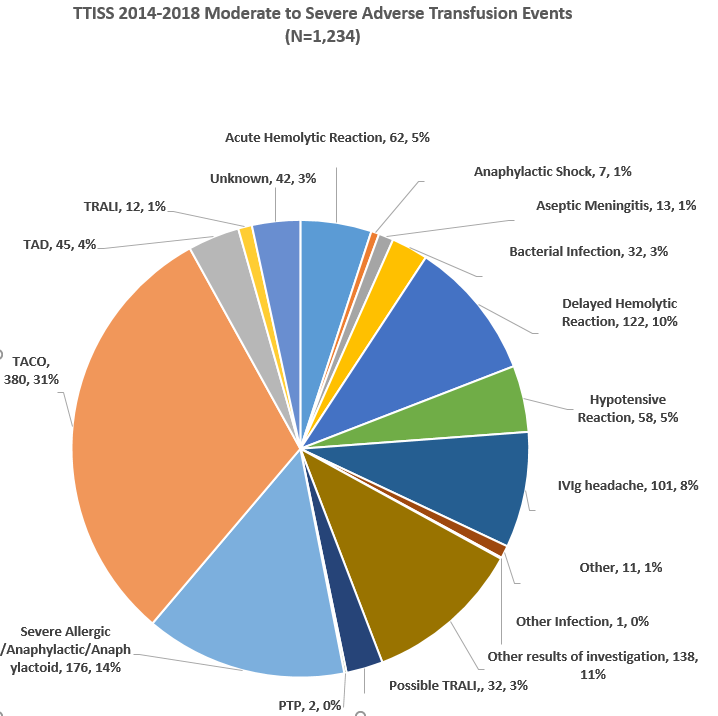
There were 1,234 reportable ATEs collected 2014-2018.
These reportable ATEs, account for 20%, of all ATEs in Ontario. The other 80% consist of minor reactions (non-reportable to PHAC). A subgroup of 28 hospitals, referred to as sentinel sites, report the non-reportable, minor ATEs (minor allergic, delayed serological and febrile non-hemolytic reactions) to Ontario TTISS.
These sentinel sites reported 43.6% of all the ATEs and 1,982 non-reportable ATEs. 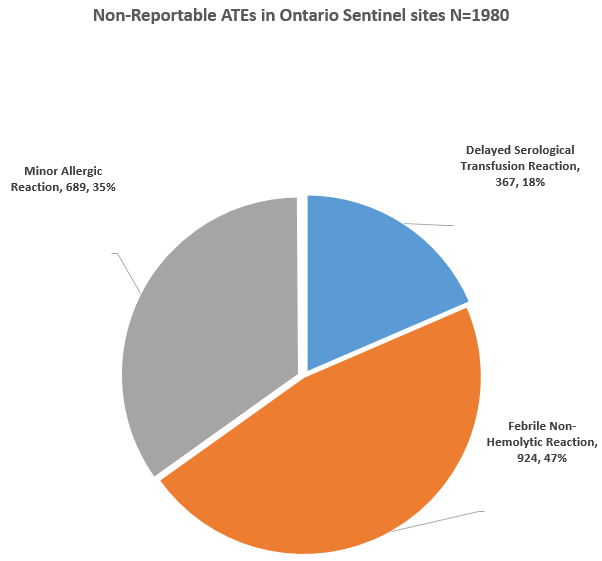
Since Ontario TTISS obtains denominator data for blood components, comprehensive reporting by sentinel sites allows risk calculation for ATEs as a result of transfusions with blood components.
Red blood cells were implicated in 632 (72.2%) of the reactions to blood components. ATEs associated with plasma derivatives were most frequently reported with IVIG (308; 87.5%).
Overall, 590 (47.8%) of the 1,234 reportable ATEs were severe or life threatening; 462 (78.3%) were related to blood components and 121 (20.5%) were related to plasma derivatives. Fifty-six deaths were reported, of which 24 were related to transfusion. Twenty-one of these deaths were associated with blood components, 3 with plasma derivatives. For more information please see our full 5 year report (2014-2020) posted on the Ontario TTISS website under transfusion data.
NEW Reporting requirements as of December 16, 2019 due to the impact of Vanessa’s Law, will not impact the regulatory requirements with respect to reporting ATEs from transfusion components. For plasma derivatives (plasma protein products) it is now mandatory to report serious reactions directly to the Canada Vigilance Program at Health Canada. forms will be accepted. For full details and rational see the NEW Ontario Guide to Reporting Transfusions Reactions on the TTISS website located under resources. TTISS